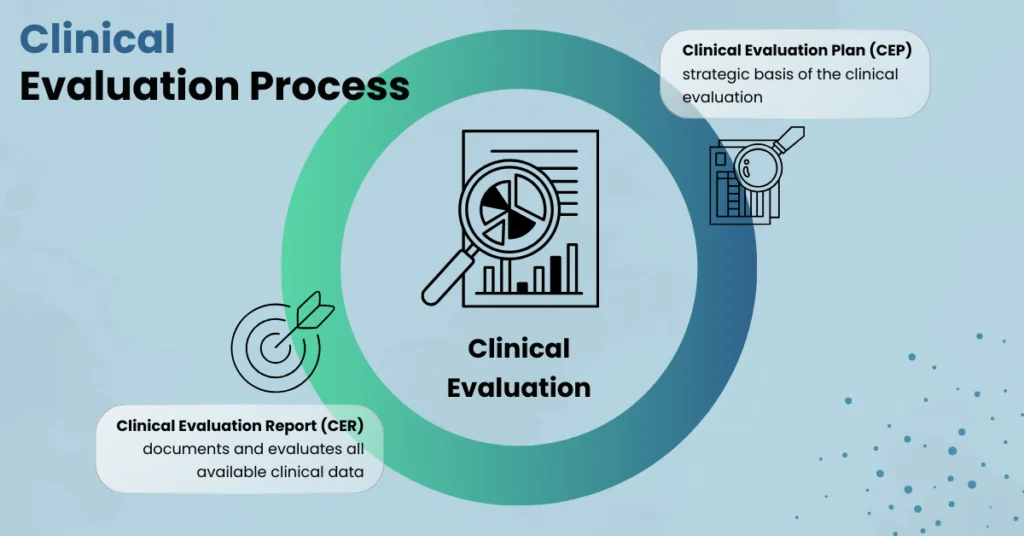
Clinical Evaluation Process according to MDR
The key function of the Clinical Evaluation Plan (CEP) and Clinical Evaluation Report (CER) The Clinical Evaluation Process is a central component of the regulatory
Welcome to our News section, where we share important updates and insights as a EU MDR Consultant regarding the Medical Device Industry, as well as company-specific news. Here, you’ll find articles covering the latest developments in CE Certification, MDR compliance, EU Regulations, and more. We also keep you informed about our own achievements, projects, and milestones, as well as how we continue to support US Medical Device Companies in their journey to the European Market.
Stay updated:

The key function of the Clinical Evaluation Plan (CEP) and Clinical Evaluation Report (CER) The Clinical Evaluation Process is a central component of the regulatory

Proposal for a regulation to simplify regulations on medical devices and in vitro diagnostic devices The European Commission has presented a legislative proposal introducing targeted

Every Christmas donation makes a difference. We will continue to support Ärzte ohne Grenzen in 2025. In the recent years, we have already decided to

New EUDAMED Registration Requirements: The Countdown Begins EUDAMED is the European database for medical devices and in vitro diagnostics. It serves as a central platform
Artificial Intelligence (AI) is redefining the MedTech industry with enabling precision, efficiency, and personalization. Yet as innovation accelerates, regulatory complexity increases. The European AI Act,

New FDA QMSR guideline! Your pathway to U.S. compliance starts here. The FDA has introduced the Quality Management System Regulation (QMSR), replacing essential elements of

Artificial Intelligence is revolutionizing medical technology. However, EU regulators expect stable, quantified proof of safety and performance for the intended use. Under MDR Annex XIV

Developing and certification of medical software The development of medical apps is a complex process that places high demands on security, functionality, and regulatory compliance.

Why Risk Management doesn’t stop after Market Launch For medical device manufacturers, achieving CE marking is a significant achievement, but it is not the end

The Certification Process in Europe relies on a decentralized model: Notified Body. These independent organizations are designated to assess the conformity of Medical Devices with

The United States is the world´s largest dynamic medical device market. Espacially for startups and SMEs it is a key step for market entry. The

Medical Device logistics is more than just warehousing. For regulated products like medical devices and IVDs, your logistics must meet strict compliance, quality, and safety

Entering the EU Medical Device Market is just the beginning. Maintaining compliance throughout your product’s lifecycle is where the real challenge begins and that’s where

For manufacturers of medical devices in the EU, a compliant Quality Management System (QMS) is not just a formality, it’s a legal requirement. Under the

Placing a medical device on the European market requires more than just an innovative idea — it demands clear proof that the device is safe,

Before a medical device can be placed on the European market, it must comply with the legal and regulatory requirements defined by the EU Medical

The WQS Management Consultants team is proud to support the recent publication from the Regulatory Affairs Professionals Society (RAPS):Fundamentals of Medical Device Regulations: A Global

WQS Management Consultants is proud to announce the launch of our U.S. entity, WQS INC, based in Florida. This new business expansion allows us to

Dear Business Partners, as the year draws to an end, we would like to thank you for the good cooperation and wish you a Merry

Under the motto “With fun instead of speed”, the focus was not on achieving personal bests, but on having fun running together. The evening started

Dear Business Partners, this year, we will again refrain from sending individual Christmas Gifts and donate an amount to a charitable organization. We have once

Certificate Extension EN ISO 13485 for a consulting company in the field of Medical Technology WQS Management Consultants GmbH is now also certified for the

Transition from the Medical Devices Act to the Medical Devices Regulation The change from the MDD (implementation of the MPG in Germany) to the MDR

WQS receives a special approval for a Covid Test for Self-Testing under the approval number 5640-S-156/21 SARS-CoV-2 Antigen Rapid Test Kit On April 7, 2021,

CE Hosting – Provision of certifications At the customer’s request, we are also able to carry out a “product certification” in our name. WQS is

Job advertisement: Regulatory Affairs Manager – Clinical Investigation WQS Management Consultants is a Consulting Firm in the field of Medical Devices – Regulatory Affairs, established

Dear Friends and Business Partners, we are happy to announce that the outdoor facilities of our new company building are now complete. We now have

Dear business partners, this year, as in the past, we are donating to a charitable organization instead of sending individual Christmas Gifts. We have once
Hamm, May 5, 2017 The official version of the Medical Device Regulation was published in the Official Journal today. This publication also applies to the

VitaSoniK® Poduction is a manufacturer of innovative medical, sports, home and cosmetic systems based in Geretsried, Bavaria. The developers of all VitaSoniK® and VitaLizeR® systems

Balda is a specialist in plastic solutions where precision, quality, safety and customized specifications are important. The group works for customers in the healthcare

Adhesive Plasters and Heart Catheters WQS Management Consultants certify Medical Products Every Medical Product, from adhesive plasters to pharmaceuticals and prostheses to complex heart catheter
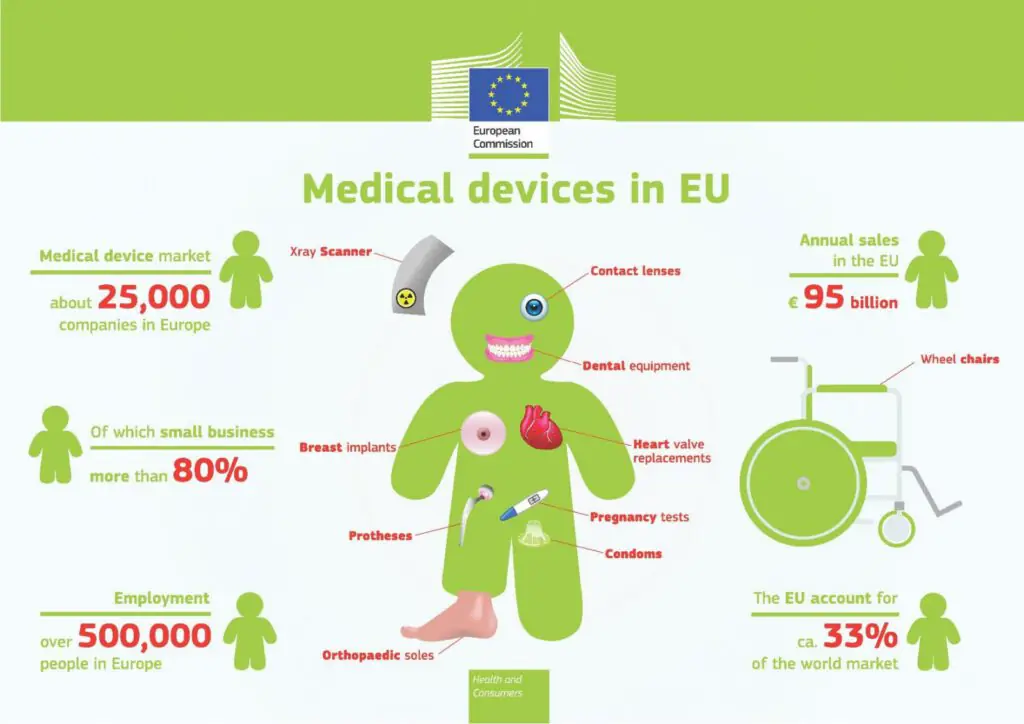
The European Commission has created a small chart regarding medical devices in Europe. Key data on market volume, employees and some basic Medical Devices are
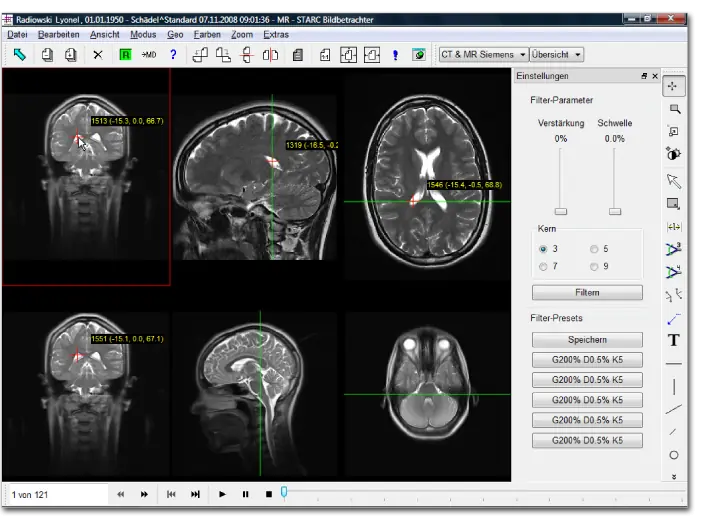
Successful Re-Certification according to EN ISO 13485 and Annex II of the Medical Device Directive | Isernhagen, October 2013 In October 2013, Starc Medical GmbH,

Quality through experience Founded in 1993, WQS – WQS Management Consultants is one of the leading companies in the field of consulting, approval, certification and
Support for US companies to enter the European market. EU Rep, Certification, QM System, Technical Documentation, support of the Procedure, selection of a Notified Body, support of the Certification Procedure, preparation of a Clinical Evaluation, support of Clinical Studies, including the Logistical Concepts.

Copyright 2024 WQS Management Consultants GmbH – All rights reserved